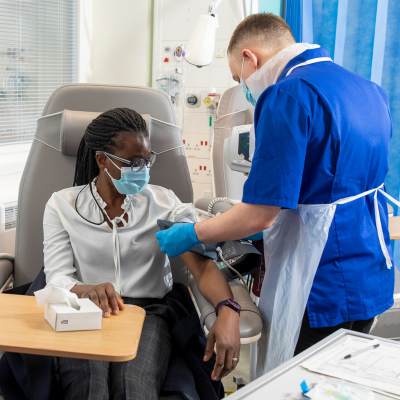
World class clinical research

Clinical research is extremely important to us, forming a key part of our tripartite mission.
Through our five-year research and development strategy, our vision is to become a top 10 research trust, achieving the best access, experience and outcomes for our patients and staff, and for our research to make a major impact in improving the care of our local populations and beyond.
We have a long history of innovating and leading the way in advancing understanding and treatment of some of the most challenging diseases. Hundreds of research studies take place across our services every year, many of which result in the discovery of new ways to treat and care for our patients.
Our research studies range from observational surveys looking at quality of life, to complex clinical trials of new medicines, surgery and medical devices.
We undertake world-leading early-stage and complex experimental studies at our specialist clinical research facility (CRF) — the National Institute for Health and Care Research (NIHR) Royal Free CRF — and collaborate with academic, clinical, charitable and commercial research organisations to enhance our research offering.
Research and development at a glance
- 200 plus research projects each year
- More than 13,000 patients involved in clinical research in 2022-2023
- 10th best NHS trust for recruitment into NIHR-adopted studies in 2022-2023
- Founder member of UCL Partners — one of seven accredited academic health science centres in the UK.
Our vision is that by 2027, the Royal Free London will be a top 10 research trust through all staff and patients having excellent access, experience, and outcomes by virtue of world class clinical research.
We aim to deliver this vision through six strategic objectives. We will:
- Provide rapid, responsive, cost effective and transparent clinical research support.
- Improve clinical research infrastructure to enable the best possible clinical research opportunities and experience to staff and patients.
- Ensure all our staff can be part of clinical research regardless of their role or site.
- Ensure optimal and equitable access to excellent clinical research to all patient groups across our local populations.
- Work with our partners to maximise the opportunities for clinical research for our patients and staff.
- Ensure that digitally enhanced and data driven clinical research is enabled throughout our clinical research endeavour.
For more information on the research strategy or to request a copy, please contact us at rf.
Research and development office
The Basement,
The Grove
Royal Free Hospital
Pond Street
London NW3 2QG
Office hours are Monday to Friday, 9am to 5pm.
Key contacts
General enquiries
Email: rf-tr.randd@nhs.net or call 020 7317 7558 (internal ext. 86316).
Amendments
Email: rf-tr.
Research applications
All theme 1 related applications (including the site contract and costings):
Email: rf-tr.theme1research@nhs.net
All theme 2 related applications (including the site contract and costings):
Email: rf-tr.theme2research@nhs.net
All theme 3 related applications (including the site contract and costings):
Email: rf-tr.theme3research@nhs.net
All theme 4 related applications (including the site contract and costings):
Email: rf-tr.theme4research@nhs.net
All theme 5 related applications (including the site contract and costings):
Email: rf-tr.
All theme 6 related applications (including the site contract and costings):
Email: rf-tr.
Our senior research team
Lucy Parker
Interim director of research and development
Email: lucy.
Derralynn Hughes
Clinical director of research and development
Email: derralynnhughes
Aderonke Adebiyi
Associate director of nursing research
Email: aderonke.
Bart Philipczyk
Research and development finance manager
Email: bart.
 Translate
Translate