
The first in-human trial of a dialyis machine for liver patients has been shown to be safe and effective, according to pioneering research carried out at the Royal Free Hospital.
The DIALIVE device, developed by hepatologist Professor Rajiv Jalan, could represent a huge breakthrough for patients suffering from organ failure due to liver disease. It works by removing toxins from the bloodstream that accumulate due to liver failure, which then allows the liver and other organs to recover.
The research, which is a collaboration between the Royal Free Hospital, UCL, pharmaceutical company Yaqrit and their collaborators, found that the DIALIVE device was safe and was associated with substantial improvement in the severity of symptoms and organ function in patients with acute-on-chronic liver failure (ACLF).
The next step will be a larger clinical trial, which if successful could see DIALIVE approved for clinical use within the next three years.
Worldwide, it is estimated that there are around 100 million people living with cirrhosis of the liver and 10 million who have cirrhosis plus an additional complication. Cirrhosis can be caused by a number of factors, including alcohol abuse, obesity, viral infection or a genetic abnormality. ACLF, a condition that can cause liver function to suddenly decline, placing individuals at high risk of short-term death, accounts for around three million individuals with cirrhosis.
The UK sees around 15,000 ACLF patients each year whose treatment costs the NHS in the region of £100,000 per patient, without improving their mortality risk.
The liver is a complex organ that performs over 500 functions, including removal of harmful substances from the blood and absorbing nutrients. One of its roles is to produce large quantities of a protein called albumin, which travels around the body acting as a sort of ‘mobile liver’ that absorbs toxic substances from the blood. But when liver function is seriously reduced, less albumin is produced which means those toxic substances remain in the blood, damaging internal organs. In addition, the liver cells can die and the gut begins to leak bacteria into the bloodstream, which can cause an inappropriate immune response, that further damages the body.
This European study is the first-in-human randomised, controlled clinical trial of a liver dialysis device. It was performed with the aim of assessing the safety of DIALIVE to treat ACLF patients and to observe its clinical effects. A total of 32 patients were treated with DIALIVE or standard care for up to five days and the outcomes were recorded at days 10 and 28.
The results showed that DIALIVE treatment was associated with significantly faster reversal of ACLF compared with standard care, with ACLF resolving in twice the number of patients compared with those who were on standard care. This clinical improvement was associated with significant impact on the mechanisms underlying the development of ACLF.
DIALIVE treatment led to a significant reduction in endotoxins, which are released when bacteria die, and improved albumin function. Biomarkers of systemic inflammation, such as endothelial function and cell death, all improved. Despite receiving as little as three days’ treatment, patients whose ACLF resolved remained in remission for 28 days afterwards.
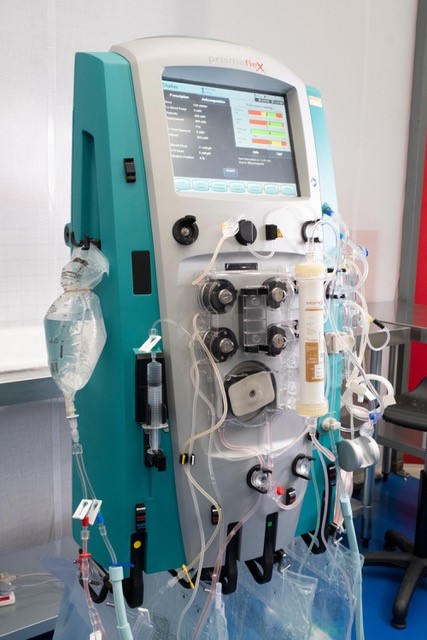
The DIALIVE device
Dr Banwari Agarwal, Chief Investigator of the DIALIVE trial at the Royal Free Hospital, (pictured on right), said: “It gives me enormous pleasure to see the promise of this novel liver dialysis device for the treatment of acute-on-chronic liver failure. The intervention has the potential to transform the care provided to the ever-increasing number of patients and their families suffering from the effects of living with what is essentially a terminal illness for many. It has the potential to transform the therapeutic options available to clinicians across the world for patients with ACLF.”
The next step will be to conduct a larger trial with more patients to confirm DIALIVE’s safety and effectiveness. One of the additional metrics to be assessed will be the impact on patient survival of DIALIVE versus other available care.
The trial is the latest step on a long journey for Royal Free Hospital and UCL researchers and their partners, that began with the identification of acute-on-chronic liver failure in 2001 as a distinct clinical syndrome that occurs in patients with cirrhosis. The intellectual property for the technology was patented by UCL in 2009 and licensed to a spin-out company, Yaqrit. Pre-clinical testing was supported by the UK government and this trial was made possible by an EU Horizon 2020 grant.
Professor Rajiv Jalan (UCL Medicine), inventor of DIALIVE and co-founder of Yaqrit, (pictured on left), said: “As academics it can be difficult to define a disease then translate this knowledge into a clinical solution that makes a real difference to people’s lives. So, the results of the DIALIVE trial are an emotional moment, which wouldn’t have been possible without scientific collaboration between the UK, European colleagues, and funding from the European Commission. My hope is that within a few years we will start to fulfil the urgent unmet need for treating acute-on-chronic liver failure and improve outcomes for patients.”
 Translate
Translate